 The ISO 8600 series outlines standards for endoscopes and endotherapy devices, primarily focusing on the performance, safety, and testing methods of these medical instruments. It covers:
The ISO 8600 series outlines standards for endoscopes and endotherapy devices, primarily focusing on the performance, safety, and testing methods of these medical instruments. It covers:
-
Performance Criteria:
- Image Quality: Standards for image resolution, clarity, and color reproduction.
- Light Source and Illumination: Guidelines for light intensity, uniformity, and safety of light sources used in endoscopes.
- Mechanical Properties: Requirements for the flexibility, durability, and strength of endoscopic instruments.
-
Safety and Biocompatibility:
- Materials: Standards for materials to ensure they are safe and biocompatible with human tissues.
- Sterilization: Guidelines for sterilization processes to prevent infections.
-
Testing Methods:
- Optical Testing: Methods to assess image quality, field of view, and depth of field.
- Mechanical Testing: Procedures for testing the physical durability and mechanical performance.
- Electrical Safety: Ensures the electrical components of endoscopes meet safety standards to prevent hazards.
-
Marking and Labeling: Requirements for clear and accurate labeling of endoscopic devices, including manufacturer information, model identification, and safety warnings.
-
Documentation: Standards for technical documentation, including user manuals, maintenance instructions, and technical specifications.
These standards ensure that endoscopes meet high-quality performance and safety criteria, providing reliable and safe tools for medical procedures.
These are the standards specifically relating to Image Quality
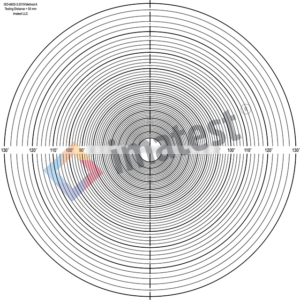 ISO 8600-3 Determination of field of view and direction of view of endoscopes with optics
ISO 8600-3 Determination of field of view and direction of view of endoscopes with optics
The angular range of observable area, in degrees, can be measured with ISO 8600-3 Method A FOV Chart.
Imatest version 24.2, which shall be released in fall of 2024, will be providing automated analysis of images of this target. If you are interested in testing this functionality please join the Imatest Pilot Program.
Automated variants of this FoV calculation can also be achieved with Checkerboard or SFRplus targets.
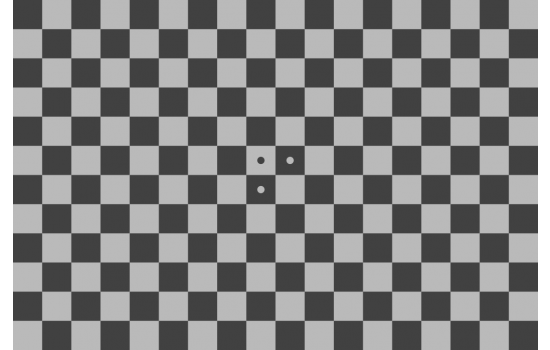
Chrome on Glass Checkerboard Target
ISO 8600-5 Determination of optical resolution of rigid endoscopes with optics
Expressed as Spatial Frequency Response (SFR) or Modulation Transfer Function (MTF), measured from slanted edges from targets such as the checkerboard, SFRplus, eSFR ISO test targets.
The standard recommends chrome on glass as the target technology to guarantee exceeding the endoscope’s resolution. For testing with this type of glossy-surface target, it is critical to disable the endoscope’s built-in illuminator. If this is not possible, a matte (Lambertian) reflective photographic checkerboard target can be used.
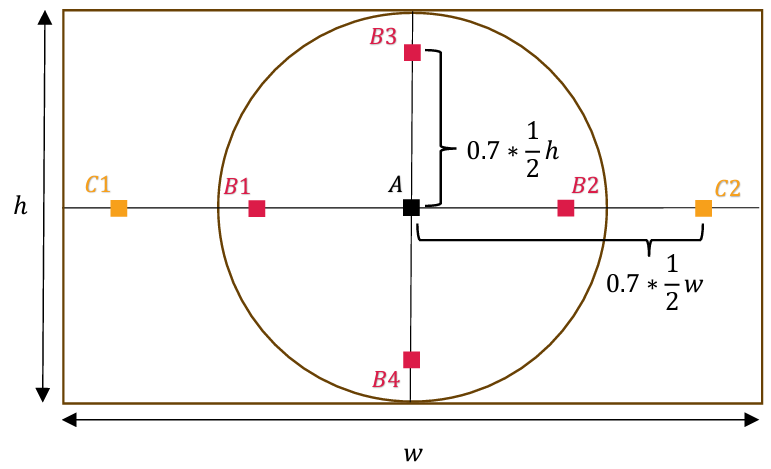 ISO 8600-5 recommends testing slanted-edge SFR from vertical and horizontal edges near 7 locations across the image. Points B1-B4 are located at 70% of the height-based radius from central point A. Additional points C1 and C2 are at 70% of the image length from the image center.
ISO 8600-5 recommends testing slanted-edge SFR from vertical and horizontal edges near 7 locations across the image. Points B1-B4 are located at 70% of the height-based radius from central point A. Additional points C1 and C2 are at 70% of the image length from the image center.
ISO 8600 Image Quality Testing Services
Imatest can provide full service endoscope Image Quality Testing Services.
- Our customized service achieves the testing objectives of your organization while working within your budget.
- Trained consultants will spend time with your team to better understand your needs and create a test plan to meet your project goals.
- Our detail-oriented engineers will test your equipment using our hardware, charts, and software to analyze images and interpret results—saving you time and resources.
- Provide consistent, repeatable, and trustworthy results through rigorous testing protocols, allowing you to build a portfolio of reports.
Endoscope Testing Equipment
The Imatest Benchtop Test stand configured with the endoscope module add-on kit and magnetic mounting plate can facilitate the positioning of your endoscope relative to the mounted checkerboard and field of view targets. The BTS’s motorization option can enable automated testing of endoscopes.
See Also




